Proton-Conducting Ceramics
Acceptor-doped Barium Zirconates (BaZrO3) or Cerates (BaCeO3) are prototypical examples of a ceramic class that allows for protonic conduction (hydrogen ion transport), rather than the more familiar oxygen ion conduction seen in ceramics such as YSZ, SDC, and LSGM. By changing the charge carrier to the more mobile proton (transport mechanism is shown in Fig. 1), similar performance can be achieved with lower temperatures. Recently, it has been recognized that these ceramics are also steam permeable under certain conditions, an additional benefit that may be exploited in new fuel cell systems or stand-alone applications.
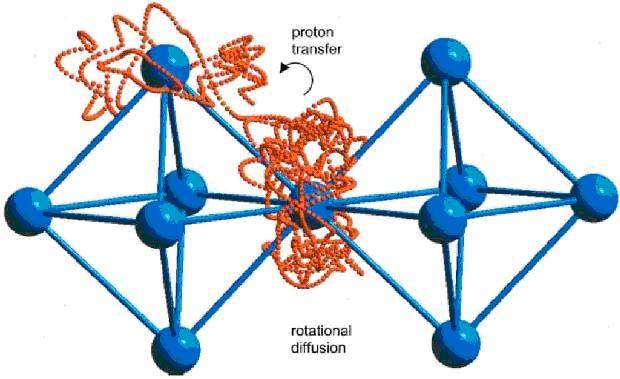
Figure 1. Grotthus type proton transport mechanism [1]
Low Temperature Solid Oxide Fuel Cells
Traditional Solid Oxide Fuel Cells (SOFCs) based on YSZ, SDC or LSGM electrolytes normally operate at temperatures higher than 600°C, which force the use of expensive materials and unique engineering challenges. However, by replacing typical SOFCs electrolytes with protonic conducting ceramics, it may be possible to achieve equal or greater fuel cell performance at much lower temperatures of 350-600°C. This temperature range gives an opportunity to incorporate less expensive and more traditional engineering materials, such as stainless steels, copper and nickel. Additional performance enhancements may be realized by incorporating ultra-thin electrolytes. Fig. 2 shows the schematic of a potential single cell based on a BaZr0.8Y0.2O3-δ (BZY) electrolyte. If the thickness can be decreased to 2μm and the grain boundary effect can be obviated, a membrane area-specific resistance (ASR) of less than 0.01Wcm2 is obtainable at 400°C [2]. At a current density of 2 A/cm2, this would result in only 0.02V ohmic overpotential loss due to electrolyte resistance, confirming the tremendous promise of these materials for low-temperature operation.
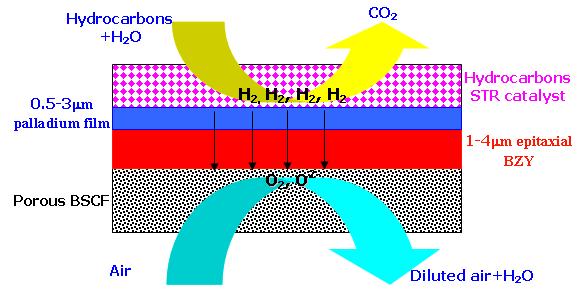
Figure 2. One potential single cell based on BZY electrolyte
A thin-film proton-conducting SOFC, such as the one shown in Fig. 2, can be fabricated using the following approach. First, a chemical solution deposition (CSD) method of electroless plating is used to deposit a thin palladium membrane on a porous substrate such as porous stainless steel, porous alumina, or porous vycor glass [3-7]. In our design, a hydrocarbon steam reforming catalyst is incorporated into the porous substrate layer, facilitating electrocatalytic reforming for operation on hydrocarbon-based fuels.
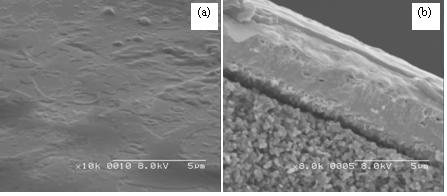
Figure 3. Pd thin film on porous alumina substrate by improved electroless plating method [7]
The thin BZY electrolyte layer can be deposited by pulsed laser deposition (PLD). The PLD method has many advantages including consistent composition transfer, epitaxial growth, lower operation temperature, versatile gas atmosphere, controllable morphology etc. The PLD method has mainly been harnessed to deposit high quality semiconductor films or superconductor films. There have been a few attempts to make thin oxide-ion conducting electrolyte films such as YSZ, SDC, or LSGM via PLD, and in most of these cases, excellent performance was obtained. These successes highlight the potential of thin-film fuel cell architectures, and so we are working on the deposition of dense, epitaxial BZY thin films via PLD.
In order to produce PLD thin-film BZY membranes, the first step is to develop a stochiometric BZY target. We are implementing a convenient, novel, cost-effective solid reaction technique to prepare pellet type targets. Starting from barium carbonate/barium oxide, zirconium oxide, and yttrium oxide as starting raw materials, a sintering additive is added and the powders are ball-milled in isopropanol solvent. Disc-shaped targets are then pressed from the ball-milled powder mixture and the green targets can be sintered directly at a relatively low temperature (~1500°C) to obtain final high quality dense targets.
At the same time, we use a sol-gel synthesis method to synthesize high quality powers using barium nitrate, zirconium nitrate, and yttrium nitrate as raw material and EDTA acid and citric acid as complex ligands. pH modification via ammonium hydroxide distributes the metal ions of barium, zirconium and yttrium at the molecular level. As water is driven out by vaporization, the solution gradually polymerizes and changes into a polymer sol and finally into a gel. After drying and calcining we obtained high quality pure BZY powder even at temperatures as low as 800°C. Moreover, we can stop somewhere during the water vaporization process and prepare a coating solution for direct deposition of BZY thin films via dip-coating, spin-casting, or spray-coating.
Steam Permeation Membrane
Steam-permeation membranes permit the transport or separation of water vapor, and have the potential to significantly improve the efficiency of a variety of energy conversion technologies including solid oxide fuel cells, membrane reformers, and coal gasification. Until recently, steam permeation membranes have been ignored because it has generally been believed that steam either could not be transported selectively, or that transport rates would inevitably be far lower than O2 transport membrane (OTM) alternatives. Recent results indicate that these beliefs are ill-founded, and fuel-reformers based on steam-permeation membranes are now a compelling alternative to OTMs. Our laboratory is pursuing an innovative strategy to design steam permeation materials by tailoring a well-known class of proton conducting membranes (which exhibit remarkably high protonic conduction) for ultra-high steam-permeation instead.
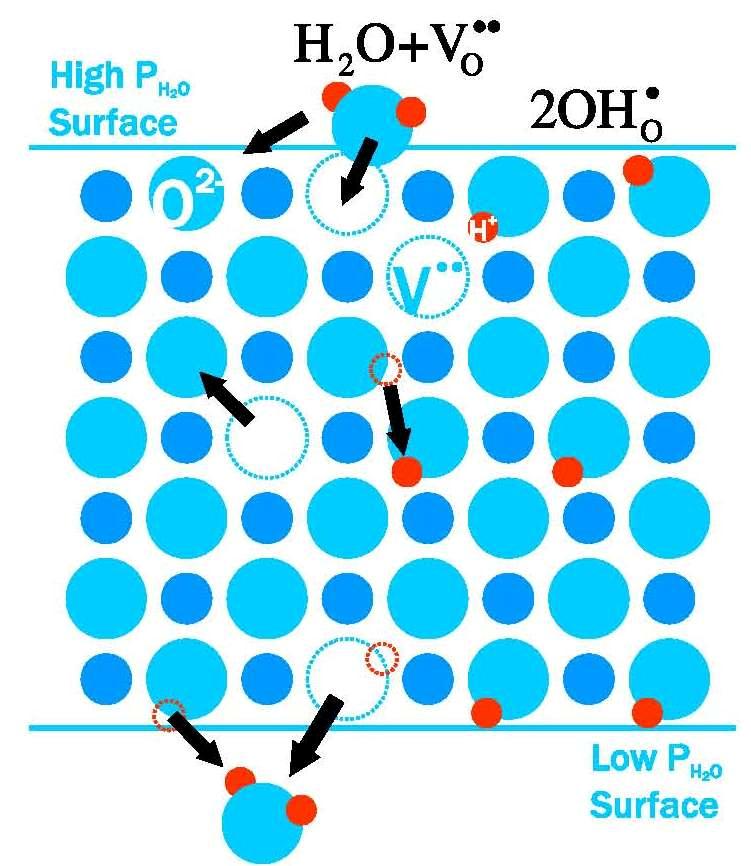
Figure 4. Steam permeation mechanism through proton conducting ceramic membrane
In particular, we are currently focusing on the steam-permeation potential of yttria-doped barium zirconate (BZY) and yttria-doped barium cerate (BCY) materials. These perovskite-based proton conducting materials have been widely investigated over the last 10-15 years for application in intermediate temperature protonic ceramic fuel cells. Recently, it has been discovered that, in addition to high protonic conductivity, these materials also exhibit impressive steam-permeation capabilities under suitable conditions. These steam separation properties have not yet been fully explored or exploited, however, and this is the focus of our current research.
Testing of the thermodynamics of steam-permeation is currently underway. The amount of steam that the membrane contains can be quantified by the change in lattice spacing, as the water is “stuffed” into the lattice. This can be readily measured using a dilatometer and measurements can be made in-situ over a large temperature range.
A test apparatus was constructed to investigate permeation kinetics. Steam introduced into a gas chamber surrounding of one side of the test sample will diffuse to a chamber on the opposite side where its gas composition can be monitored by mass spectrometry. By alternating between water vapor produced with water and heavy water (D2O) the rate of permeation can be quantified for various temperatures.
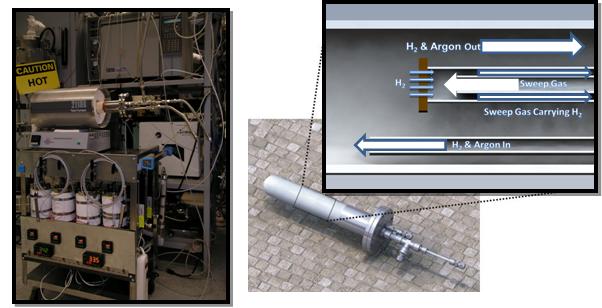
Figure 5. Steam permeation test setup and permeation cell
Testing procedures are being developed to quantify the performance reduction of BCY10 and other steam-permeable ceramics due to degradation caused by the presence of carbon dioxide, carbon monoxide, as well as water. It is understood that these environments lead to a degradation of ceramic powders. However, it is only theorized that this phenomenon is not extended to highly-densified solid membranes. The goal of this research is to explore this theory and possibly quantify what loss in performance may occur, if any at all.
References
- W. Miinch, G. Seifert, K.D. Kreuer, J. Maier, Solid State Ionics 86-88 (1996) 647.
- K.D. Kreuer, Annu. Rev. Mater. Res., 33 (2003) 333.
- S. Uemiya, N. Sato, H. Ando, T. Matsuda, E. Kikuchi, Appl. Catal. 67 (1991) 223.
- S.N. Paglieri, J.D. Way, Innovations in palladium membrane research, Sep. Purif. Methods 31 (2002) 1.
- J.H. Tong, Y. Matsumura, Chem. Commun. (2004) 2460.
- J.H. Tong, H. Suda, K. Haraya, Y. Matsumura, J. Membr. Sci., 260 (2005) 10.
- J.H. Tong, L.L. Su, K. Haraya, H. Suda, J. Membr. Sci., 310 (2008) 93.